Un article de Isabelle Moretti dans une revue web
The history of energy is one of gradual substitutions from inefficient, dirtier, expensive options to cleaner, cheaper, higher-performing fuels. Mills and machines replaced manual labor, and more recently electricity replaced kerosene, which had replaced whale oil for lighting, and coal replaced wood for industry and heating buildings. But what about gases?
A century ago, town gas was manufactured by burning coal, producing coke and a blend of methane and hydrogen but also toxic gases such as CO and other pollutants along the way. Later, large reserves of natural gas (primarily composed of methane) were found, which were both cheaper and cleaner, so we stopped manufacturing town gas. As a result of methane’s utility, abundance and affordability, it is used for just about every sector of society. Today that gas is used for heating, cooking, power generation, and as a feedstock to make materials such as chemicals and plastics.
So what will replace fossil reserves of natural gas? Electricity can replace some uses of gas, but not all of them. Biogas is a useful alternative, but limited in scale to replace the entirety of our needs for gaseous fuels and, in some countries, it is leading to a land use debate. That means we still need some fuel that is cleaner and cheaper than gas.
The popular candidate grabbing today’s headlines is hydrogen. It burns more cleanly than natural gas, but to date has been much more expensive to manufacture from water or hydrocarbon sources.
Hydrogen: Uses and Problems
Hydrogen has until now primarily served as a raw material for industry. It is also gaining popularity as an elegant way to store electricity, but the economics of these transformations, converting electricity to hydrogen (via electrolysis) and back to electricity through fuel cells, turbines or engines (known end-to-end as Power-to-gas-to-power, or P2G2P) is difficult. Though hydrogen gained notoriety in a 2003 State of the Union speech by President George W. Bush as a transportation fuel, the competition from electric vehicles has dominated investment budgets by major automotive manufacturers, it is now quickly changing in Asia where China and Korean car manufactured get focused on H2 cars.
In 2018 there were just over 70 million tons of Hydrogen consumed for all purposes, mostly to make ammonia for fertilizers and to lighten and sweeten crude oil at refineries. Demand for hydrogen is expected to grow 8x to satisfy over 550 million tons of demand in 2050, again as a feedstock, but also for transportation, building heat, and power generation.
Unfortunately, today’s methods for producing hydrogen emit CO2 or require significant energy inputs or both. A majority of hydrogen consumed today is made from methane, or more generally from hydrocarbons, by steam reforming, a production method that emits CO2. One can also crack methane (CH4) to black carbon and hydrogen in the absence of oxygen with a method known as pyrolysis, using plasma technologies that also require heat or electricity. Hydrogen can also be produced by electrolysis, which is the process of using electricity to separate hydrogen from water.
Less than 5% of the H2 produced today is with this method. But that electricity for pyrolysis or electrolysis is not a source but an energy vector: electricity relies on the availability of a primary energy source.
Another Option: Natural Hydrogen
Though primary wind and solar energies are unlimited, they still need many natural resources extracted by mining or quarrying to be transformed into electricity. Many metals mandatory for solar photovoltaic and wind technologies, as for electrolyzers, are only produced in a few countries, making them strategically critical resources. Finding a new way to produce H2 that doesn’t emit CO2, doesn’t rely on strategic materials, and is produced more regularly than what variable sources can provide is therefore important and would be of great value.
Thankfully, there is another option that has not garnered much attention: natural hydrogen (also known as native hydrogen) that is generated by geological processes. Emanations of Hydrogen have been observed in many places. As a consequence, subsurface accumulations of hydrogen drilled “par hazard” and its direct extraction, although still anecdotal today, is beginning to be seriously considered as an abundant source of truly green and inexpensive H2 (Prinzhofer and Deville, 2015; Moretti, 2019).
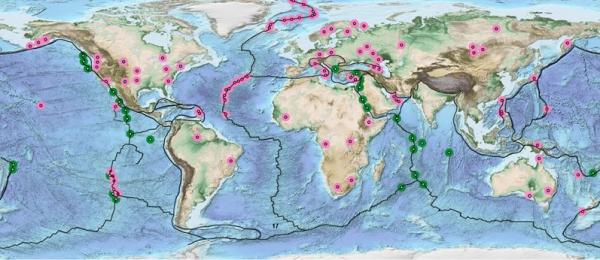
Map of the already known H2 and CH4 derived from H2 emission
The Origin of H2
Hydrogen is the most common molecule in the universe. However, in the Earth’s atmosphere it exists only in very small quantities at around 500 parts per billion (or 0.5 ppm). Other than trace amounts of gaseous dihydrogen (H2) at the Earth’s surface and above, we find hydrogen essentially combined: with oxygen in water (H2O) and with carbon in all hydrocarbons (CH4, C2H6 …). However, what is becoming clearer with time is that several phenomena lead to a continuous generation of H2 in the Earth’s crust. A water-rock interaction known as diagenesis releases hydrogen from water during oxidation phenomena that can be observed in different geological contexts. As soon as there is, for example, ferrous iron (Fe2+), in contact with water (sea or rain) it oxidizes to ferric Fe3+ and releases H2. The same reaction can also take place with other metals such as magnesium (Mg2+ => Mg3+); it is fast and efficient at high temperatures, around 300°C, but also possible at lower temperatures. Other sources of natural H2 are known. Another production pathway is radiolysis, by which H2 contained in water is separated from oxygen by the natural radioactivity of the earth’s crust (Sherwood et al., 2014). Estimates of the flow of H2 through the latter two sources, diagenesis and radiolysis, are important but still not very precise, varying according to the authors from a few percent to 100% of the annual consumption of H2 in 2019, or approximately 70 Million of tons. Other sources such as friction on the fault planes and the activity of certain bacteria also release H2 but, a priori, in smaller quantities (Worman 2020). What is important to note is that in all these cases it is a flow of H2 and not an accumulated, fossil resource. At the same time, the preservation of large quantities of primordial H2, the H2 present at the initiation of the solar system, in the mantle, or even in the earth’s core during the formation of the earth is also a working hypothesis explored by some researchers (Larin et al., 2015, Zgonnik, 2020). In this hypothesis, H2 is a fossil resource but almost infinite.
Where do these reactions occur? And can H2 accumulate in the subsurface?
The minerals in the rocks emitted by underwater volcanoes of the mid-ocean ridges, especially olivine, oxidize on contact with water and release H2. At the level of the smokers of the mid-Atlantic ridge these emanations have been studied for a long time, in particular to understand the appearance of life on earth. Some authors even made calculations on the economics of the recovery of this H2, offshore and at great depths (Charlou et al., 2002, Goffé et al., 2013). Natural hydrogen recovery from the mid-Atlantic ridge did not attract much business interest at the time that work was published because the conditions – such as water depth and distance from the coast – were considered too difficult for economic capture and transportation to market despite the large quantity of H2 released by the smokers. Those difficulties remain unsolved, so we anticipate a nascent H2 E&P industry, like that of all other natural resources, will likely start onshore. Fortunately, this type of volcano can also be observed where the mid-oceanic ridges outcrop, either because they are in an early stage as in the Afars, the triple point between the central axes of the Red Sea, the Gulf of Aden and the East African Rift, or because they are uplifted by deeper phenomena (a hot spot) as in Iceland. In fact, in this island, the fumaroles of the neo volcanic zone of the central axis of the rift all contain H2 (Stefansson, 2017). For the moment only the thermal energy content of the hot water, the heat-transfer fluid that brings energy to the surface, are used in the geothermal power plants, but it could be otherwise as those geothermal fluids contain large fractions of hydrogen. Generally speaking, production of H2 by surface separation in addition to extraction of geothermal energy would be possible in many areas such as Tosacani. This path seems to be worth exploring because the difficulties encountered in trying to make many high temperature geothermal projects economical mean that a second revenue stream from hydrogen sales would be appealing. Oceanic crusts that can oxidize are also found at or near the surface in suture zones, where the compression and the thrusting of the sheets form mountains. Oman and the Philippines are the most studied cases but H2 emanations have also been noted in New Caledonia and in the Pyrenees. Often this hydrogen reacts immediately with the CO2 in the atmosphere and precipitates as carbonate, which effectively makes the process a natural and spectacular carbon capture process.
There are other on-shore geological sources of H2 that are easier to access: Precambrian cratons that are more than ½ billion years old. A recently published synthesis by Zgonnik (2020) catalogs hundreds of cratons where hydrogen flows have been observed, including in Russia (around Moscow), the USA (South Carolina, Kansas), and also in many other places. The source could be relatively similar, namely the oxidation of an iron-rich material and the release of H2. This mechanism seems reasonable as surface leakages are systematically in zones where the basement is very old and rich in metals.
An Example of Natural Hydrogen Production
One example of natural H2 production is particularly compelling. In 1987 a well was drilled in Mali to search for water. The well turned out to be dry, but unexpectedly produced significant volumes of H2. Aliou Diallo, the director of Petroma (now renamed Hydroma) saw the possibility of local, carbon-free energy in a country that is deprived of it, so the company put the native H2 into production. The well was unplugged in 2011 in order to use it for a pilot to generate electricity for a small village. The hydrogen that comes out of the well is almost pure (more than 96%) so it can be directly burned in a gas turbine. Other surrounding wells have been drilled by Hydroma since 2018 to try to determine the size of the reserves, similar to the early years of oil & gas, and to increase the flows of hydrogen that could be used as feedstock for an ammonia production plant. Part of the results have been published, Prinzhofer et al (2018), and show that all the wells have H2 fluxes. This success has shattered many “a priori”. As of this writing in 2020, the initial well has been producing for 4 years without any pressure decrease from its initial baseline of approximately 4 bars, which implies continuous recharging of the reservoir 110m belowground. The surface measurements of the H2 sensors do not show any leakage, which leads to the conclusion that, contrary to what had been expected given the size of the H2 molecule and its ability to chemically recombine, there are seal rocks that enable an accumulation of H2 and that it can remain in the gaseous phase under our feet. Mr. Diallo and his team have done a lot to draw attention to this basin, especially since H2 can be produced there at much less than a dollar per kilogram, which is significantly cheaper than conventional costs for hydrogen production by electrolysis or steam methane reforming with carbon capture. Unfortunately, because of the complicated above-ground political and security situation in Mali, the follow-on work by the scientific community in this location essentially stopped.
Nevertheless, the production data over several years in combination with the search for a low-carbon energy sources has revived interest in the subject and various research and exploration projects have been launched since 2018 (Gauchet 2020). An exploration company dedicated to hydrogen was created in the USA (NH2E) and drilled a first well in Kansas at the end of 2019. In France the company 45-8 is looking for helium and H2, which are often co-located underground. Helium gas has strategic importance and commands a higher price than H2, so exploration and production companies often prioritize helium even though the helium market in volume is smaller than the hydrogen market. That is actually an advantage for the natural hydrogen market as companies looking for helium are likely to find hydrogen even if that was not their goal.
The Fairy Circles
When some say resources, others think reserves. And some even want to know the proven reserves before starting any H2 exploration business. Our world of the 21st century advocates innovation but is also becoming in many contexts more and more anti risk… Fortunately, our ancestors did not wait to calculate the world’s iron reserves before moving into the Iron Age.
As it stands, we do not know how much H2 is produced daily on earth by the pathways listed above. We also do not know how much of this H2 accumulates in reservoirs where it would be easy to produce it. And, perhaps, we have not yet identified all the reactions that would produce H2. After more than a hundred and fifty years of drilling, oil reserves continue to evolve constantly – in fact they continue to grow as we find more oil– and we had no idea what a source rock or an oil system was during the first 50 years of this industry. For H2 we still lack knowledge and there are very few wells dedicated to its exploration, so it is difficult to estimate total global volumes.
However, there are surface emanations that give us a hint of what to expect. What do they tell us?
Southeast of Moscow, Larin and his co-authors (2015) noted slight depressions that were roughly circular and clearly visible on aerial photos; the community called them fairy circles. Often the vegetation dies at these circles and if one goes there with a gas detector escaping H2 in non-negligible volumes can be measured in a non-constant and non-continuous way. In the USA, it is the IFPen teams that have made the measurements and the results are similar (Zgonnik et al., 2015). In Brazil, Canada, Australia and Namibia, similar features are also observed. However, to draw conclusions on the possibility of producing this hydrogen economically, it is necessary to know the volumetric flow rates and not just the concentration.
H2 sensors are available on the market that can provide a punctual measurement of hydrogen in the soil at a given moment. Engie’s research teams, aware of this need for additional data to estimate the flux and thus, eventually, the reserves, developed a new permanent sensor (Moretti et al., 2018). The H2 soil concentration is measured every hour and the data are sent directly by satellite to the researchers. More than a hundred of these sensors have been installed in the San Francisco Basin in Brazil where significant percentages of H2 in the subsurface had already been found and where witch rings (another name for ‘fairy circles’) were visible. By late 2020 they had been in operation for almost 2 years and the first published results confirm the significant, but not continuous and non-constant flow of H2 over the structure (Prinzhofer et al., 2019; Moretti et al., 2020). The integral of the measurements are roughly the same order of magnitude as that published in Russia, about 7000 m3/day, i. e. 680 kg on a 0.4 km2 structure. Importantly, this first continuous recording on a fairy circle revealed that the flow varies during the day, in a systematic way. The pattern begins with a very high pulse of H2 followed by a regular H2 flow on a cycle of 24 hours. This cycle had already been noted by those who study H2 near active faults in the framework of risk prevention but its implications had not been taken into account to our knowledge. These daily variations call into question previous data that indicated these circles were dead structures as it is possible to monitor at the wrong time when the features appear to be asleep. Thus, continuous monitoring might be an essential element of assessing and producing natural hydrogen.
The emanations that we measure in Russia, USA or Brazil are between 50 and 1900 kg/km2/day. To give perspective, with 5 kg we fill the reservoir of a fuel cell vehicle such as a Toyota Mirai. It is worth noting that geologists do not determine the volume of oil reserves by looking at the surface index, as it is only a tiny percentage that escapes, so perhaps using surface leaks of hydrogen are similarly error-prone.
Transport and Accumulation
Two other important research questions include the mode of transport of H2 in the subsurface and the conditions of its accumulation, i.e. are there rocks impermeable enough to be a seal for an H2 reservoir? H2 is a light gas and in gaseous form it may only migrate vertically. Recent joint work between ENGIE and IFPen has nevertheless challenged this assumption about its gaseous form, finding that even though H2 is not very soluble in water at shallow depth, it becomes quite soluble when the temperature and especially the pressure increase (Lopez et al., 2019). At a depth of several kilometers, H2 can thus move in dissolved form in aquifers and can thus be found far away and even laterally from its source and not just above it. In Mali the caprock is dolerite, which is a very impermeable volcanic rock, but accumulations have also been found under clays and there are industrial underground H2 storage sites in saline cavities and aquifers. Thus, a range of reservoirs and seals can exist for H2 even though there is no evidence to date that these seals would remain impermeable over millions of years like those of oil fields.
For production and distribution, the problems will be the same as those for manufactured H2 and the industry as a whole is working to develop solutions for storage (especially underground) and pressurized transport and distribution. Liquefaction, which is efficient for methane, and which allows transport by ship and a global market, is more expensive and inefficient for H2. The compression is exothermic and the temperature must be very low, so with state-of-the-art capabilities as of late 2020, it is possible to lose about a third of the energy of hydrogen by liquefying it. Because of the difficulties moving hydrogen, it is to be expected that the economy of small fields close to the consumer will be appealing, which is similar to what the example in Mali demonstrates. Furthermore, the notion of « small fields » could turn out to be different from the world of oil & gas since recharging is, according to what we know today, continuous. We may just have to check the speed of this recharge and whether production can be stimulated (for example by injecting water downhole) and adapt production to it.
Conclusions
Overall, hydrogen is an appealing low-carbon fuel that can be used for heat, transportation, power generation, and manufacturing chemicals or other materials. Conventional methods for manufacturing hydrogen are CO2-intensive or expensive. Therefore, large-scale, clean, affordable and natural sources of hydrogen from geological processes are very attractive and might solve several problems simultaneously. However, this field of study is relatively new so we should not pretend to have a perfect understanding of the system. Nevertheless, available data to date converge towards the concept of continuous production (over years) in significant quantities. Since we now know that hydrogen, in industrial quantities, is produced every day by the water-rock interaction, and that it escapes, its production seems to depend only on us; now we have to determine the most promising locations and according to the context either to separate it on the surface in the geothermal flows or to drill and stimulate the reactions. In parallel with this prospecting, an evolution of the mining law to classify native H2 will be necessary since in some countries it does not yet fall into any category allowing to apply for an exploration or production permit. Overall, our latest data and understanding suggest that natural hydrogen is available at globally-relevant volumes with potentially easier and cheaper accessibility and lower emissions, which means it could be the dominant primary energy source we need for a low-carbon future.